Executive summary
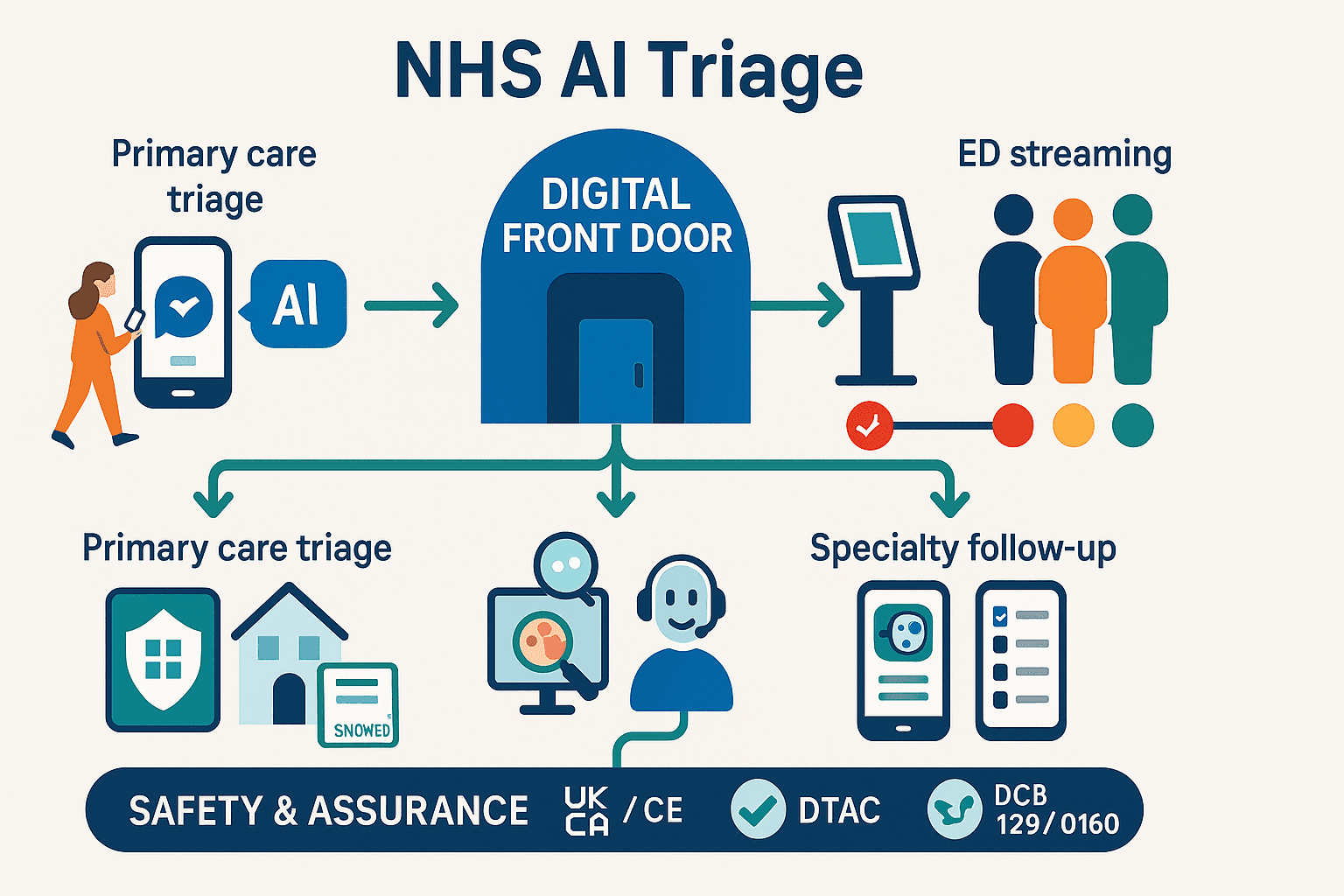
In 2025, the need to safely automate the "digital front door" for primary and urgent care has made NHS AI triage a critical area of innovation. A new generation of AI-enabled tools is helping to redirect patient demand, surface clinical risk earlier, and shorten waiting times, with a growing number of validated deployments across UK healthcare pathways. However, this is a regulated space. For UK buyers, any Software as a Medical Device (SaMD) must meet the stringent rules set by the MHRA and pass the NHS DTAC procurement baseline.
This guide provides a practical overview of the key players in the 2025 UK market. This includes primary care online triage tools like Anima, PATCHS AI, and Klinik; emergency care streaming with eConsult eTriage; and specialty-specific AI from Ada, Symptoma, and Ufonia. We will explore what they do, the evidence for their impact, and the crucial regulatory distinctions—such as MHRA device classification—that every commissioner must understand.
How to buy triage AI safely (a UK primer)
- MHRA & UKCA/CE Marking: In the UK, software that performs a medical function—like triaging symptoms to recommend a course of action—is legally a medical device. Its classification depends on the risk it poses. Many triage tools are Class I, but if a tool "allows direct diagnosis," it may be pushed to a higher Class IIa risk category, requiring more rigorous assessment. All devices must have the appropriate UKCA or CE marking.
- DTAC (Digital Technology Assessment Criteria): This is the mandatory NHS baseline for procurement. It assesses a tool against five key domains: clinical safety (including DCB0129/0160 standards), data protection, technical security, interoperability, and usability.
- MHRA AI Airlock: For truly novel or adaptive AI medical devices, the AI Airlock provides a regulatory sandbox to de-risk evidence generation before a wide-scale rollout.
Comparison table
| Vendor | Setting | What it does | Evidence Snapshot | Declared Device Status |
|---|---|---|---|---|
| Anima Health | Primary Care | Total digital triage with AI assistance | NHS rollouts, productivity case studies | MHRA Class I (claimed) |
| PATCHS AI | Primary Care | AI-powered online consultation & triage | University spin-out, published evaluations | MHRA Class I, UKCA (claimed) |
| Klinik Access | Primary Care/PCNs | AI-assisted triage & patient flow | York PCN study shows ~20% task reduction | CE-marked (claimed) |
| eConsult eTriage | ED / UTC | Digital triage & prioritisation at check-in | Vendor reports faster streaming | Check at procurement |
| Ada Health | Enterprise / Public | Symptom assessment & navigation | EU-MDR Class IIa (Ada Assess) | Check UKCA/MHRA for GB |
| Symptoma | Consumer & Pro | Medical-device symptom checker | Markets as medical device | Check Class & UKCA/MHRA |
| **Ufonia (“Dora”) | Specialty Follow-up | Voice AI for post-op triage | Imperial College pilot showed efficacy | Check Class & UKCA/MHRA |
Anima Health — total digital triage (primary care)
- What it is: An integrated online consultation and triage platform designed for GP and ICS workflows.
- Evidence & deployment: The platform is in use across a growing number of NHS sites, with case studies highlighting its impact on practice productivity and triage efficiency.
- Regulatory status (vendor claim): Anima states it is MHRA Class I registered and is pursuing Class IIa for its ambient AI components.
PATCHS AI (Advanced) — AI-powered GP triage
- What it is: A digital front door for general practice that uses AI to triage incoming requests. It also features a virtual telephone assistant to ensure parity of access for non-digital patients.
- Regulatory/compliance (vendor claim): The PATCHS website states it is MHRA Class I, UKCA marked, DTAC compliant, and holds DCB0129, Cyber Essentials Plus, and ISO 27001/9001 certifications.
Klinik Access — AI-assisted triage & navigation
- What it is: A CE-marked algorithm that classifies patient requests by clinical type and urgency, then directs them to the most appropriate service, including self-care.
- Evidence: A study in York PCN reported an approximate 20% reduction in tasks for the practice team after the workflow was integrated with Klinik.
eConsult – eTriage (ED/UTC)
- What it is: A self-check-in system for emergency departments and urgent treatment centres. It uses kiosks or tablets to capture a structured patient history and prioritise them by clinical need to speed up the streaming process.
- Evidence signal: The vendor reports that the tool can be completed in 3–4 minutes, leading to the earlier identification of sicker patients and reduced overall waiting times.
Ada Health – Ada Assess
- What it is: A symptom assessment and care navigation engine that can be embedded into partner applications and websites to act as a digital front door.
- Regulatory: Ada announced EU-MDR Class IIa certification for its enterprise assessment tool in December 2022. Any UK deployment would require confirmation of its UKCA/MHRA registration status.
Symptoma — medical-device symptom checker
- What it is: A differential diagnosis generator that takes symptom inputs, positioned for both patients and clinicians.
- Regulatory: The platform markets itself as a medical device. For any NHS use, the specific device class (I vs. IIa) and its UKCA/MHRA registration status would need to be verified.
Ufonia – “Dora” — voice AI for post-op follow-up
- What it is: An automated telephone follow-up service. The AI, named "Dora," autonomously calls patients after procedures (initially in ophthalmology), asks a series of clinical questions, understands their responses, and flags any patients who require a review from a human clinician.
- Evidence: A pilot with Imperial College Healthcare reported that the service provided enhanced post-cataract care and accurately identified the patients who needed clinician review.
Feature checklist: what “good” triage AI looks like
- Clinical provenance & safety: Does it have clear red-flag logic and "seek help now" behaviours? Is there a full audit trail?
- Regulatory status: Does it have a declared MHRA class, a UKCA/CE mark, and a public registration?
- DTAC evidence: Is there a complete, current, and satisfactory DTAC pack?
- Integration: Can it hand over information cleanly into your EMIS, SystmOne, or acute EPR, ideally with structured, coded data?
- Equity & access: Does it support multiple channels (e.g., telephone for non-digital users), languages, and meet accessibility standards?
- Measurement: Can you track time-to-triage, safe deflection rates, and re-attendance or safety events?
FAQs
- Are online triage platforms always medical devices?
- If they perform a medical triage or assessment function by interpreting patient-specific data, they are generally considered to be medical devices under UK law. The classification (risk level) depends on their specific claims and function.
- What’s the difference between Class I and IIa for these tools?
- A higher risk Class IIa may apply if the tool's output allows for a "direct diagnosis" or provides decisive diagnostic information that is not easily verifiable by the user. Otherwise, many triage and assessment tools fall into Class I.
- Is DTAC the same as MHRA approval?
- No. The DTAC is an NHS procurement baseline covering a range of standards. MHRA registration and UKCA/CE marking govern a product's legal right to be placed on the market as a medical device. For NHS use, you will typically need to evidence both.