Executive summary
The rapid rise of artificial intelligence in healthcare has created a new, high-demand, and scarce commodity: the clinical expert. As digital health moves from pilot projects to regulated deployments, the need for clinician input is being codified into law and practice. UK governance frameworks like the NHS DTAC, the MHRA’s AI Airlock for medical devices, and NICE’s Early Value Assessment (EVA) pathway all mandate rigorous clinical oversight.
This has created a new economy for clinician expertise that goes far beyond traditional locum work. Demand is soaring for doctors, nurses, and pharmacists to act as paid advisors, evaluators, and safety officers. Rates for expert network calls can range from £120-£250+/hour, while formal roles like Clinical Safety Officer (CSO) offer structured, high-impact contract work. This guide provides a practical map of 12 routes to monetise your expertise and introduces iatroX Insights, a new clinician-first advisory arm designed to channel paid projects from the industry directly to practising clinicians.
The landscape: why clinicians are economically valuable to AI
A software developer can build an AI, but they cannot tell you if its output is clinically safe, relevant, or useful in a busy NHS clinic. This "last mile" of clinical validation is the most valuable part of the health-AI chain. The UK's new assurance stack—DTAC for procurement, the AI Airlock for novel AI, and NICE EVA for evidence generation—formalises the need for this clinical judgement at every step. Your expertise is the critical ingredient that turns a clever algorithm into a safe, adoptable medical tool.
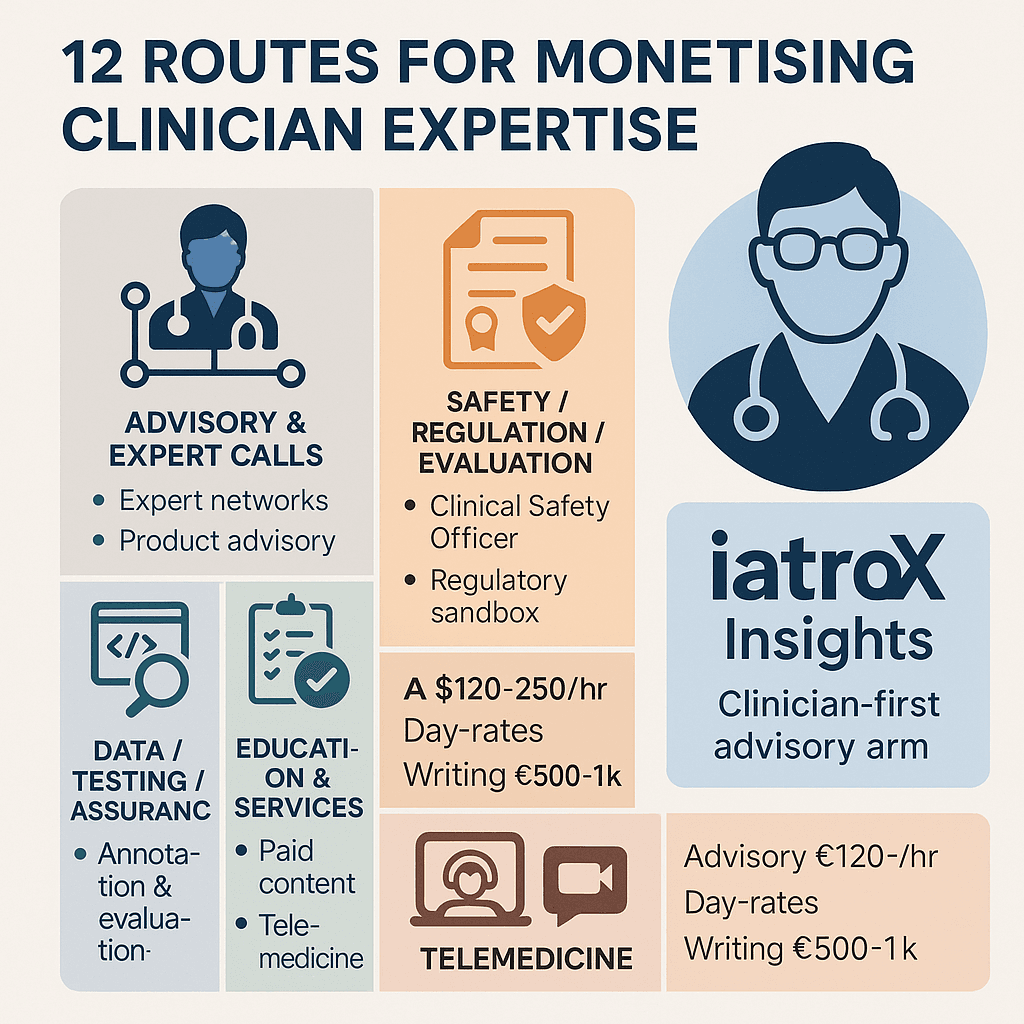
Monetisation pillars (12 routes, UK-relevant)
A. Advisory & expert calls
- Expert networks (e.g., GLG, Maven): Investors and tech companies pay high hourly rates (often £120–£250+) for 30–60 minute phone calls to understand a clinical pathway, a guideline, or the procurement landscape.
- Direct product advisory boards: Start-ups and established vendors will pay for your time to sit on their advisory boards, helping them validate their product roadmap and avoid building features that clinicians don't need or want.
B. Safety, regulation & evaluation
- Clinical Safety Officer (CSO): A formal, paid role. Under the NHS's mandatory DCB0129/0160 clinical safety standards, all health IT products and the organisations deploying them must have a registered clinician (the CSO) to sign off on the clinical risk management file, including the safety case and hazard log.
- Regulatory sandbox participation: Clinicians are needed to help design and run pilots for novel tools in the MHRA AI Airlock, generating the real-world evidence that informs future regulation.
- NICE Early Value Assessment (EVA) ecosystem: NICE's faster assessment pathway for digital tech relies on expert panels. These roles often come with expenses or honoraria and place you at the centre of national-level evidence evaluation.
C. Data, testing & assurance
- Clinical data annotation & model evaluation: This is the fast-growing market of "red-teaming" AI. Companies will pay for your clinical expertise to review an AI's outputs, label its errors, and test it for bias and safety issues.
- Protocol design & outcomes mapping: Designing the evaluation protocol for a new AI pilot is a highly skilled task. Clinicians are paid to help define the endpoints that matter (e.g., time-to-diagnosis, referral accuracy) in a way that aligns with NICE EVA expectations.
D. Education, content & communities
- Paid clinical content: Platforms (including some like Geeky Medics) commission clinicians to write or review OSCE guides, videos, and exam question banks.
- Medical journalism & features: Reputable outlets like the BMJ or Health Service Journal, as well as health tech publications, commission features from clinical experts.
- Accredited learning products: Co-creating a CPD-accredited module for a provider is a high-value skill, blending your clinical knowledge with educational theory.
E. Services & clinics (AI-adjacent)
- Telemedicine & remote consults: The rise of digital-first providers has created a huge, flexible market for remote clinical sessions that can be balanced alongside other advisory work.
- Medicolegal & expert witness work: A highly specialised and well-remunerated field with structured fee scales, requiring specific training, indemnity, and adherence to disclosure rules.
iatroX Insights: a clinician-first pipeline for paid projects
We built iatroX to support clinicians. Now, we want to help you leverage your expertise.
- What it is: iatroX Insights is the new advisory, research, and partnership division of iatroX. We support NHS partners and digital-health companies with evaluation, clinical safety, and adoption strategy.
- How it pays back clinicians: We are building a community of clinical experts. When a paid project comes to us—an evidence review, DCB0129/0160 safety input, a design sprint, or an AI red-teaming exercise—we route that work (and the payment for it) to practising clinicians within the iatroX community.
- Our synergy: We use our own platform to work smarter. The iatroX Knowledge Centre, with its rapid search of UK-accepted guidance and peer-reviewed research, helps our clinical experts underpin their evidence-based tasks efficiently.
Pricing your time
- Advisory calls: Market guides show expert networks frequently pay clinicians $150–$300+ (£120–£250+) per hour.
- Project work: Day-rates for a qualified Clinical Safety Officer or clinical reviewer should reflect the seniority and risk of the role, often benchmarked against consultancy rates.
- Writing & education: Commissioned features for major outlets can pay £500–£1,000, while platform-specific contributor fees vary.
- Expert witness: Use the published official schedules as your anchor and price based on complexity, court time, and report preparation.
Governance & ethics (protect your licence while you earn)
- Declare conflicts of interest when joining any committee, publishing, or advising.
- Insist on a DPIA (Data Protection Impact Assessment) and robust anonymisation if you are asked to review or annotate any real patient data.
- Anchor your advice in trusted, verifiable UK sources like NICE guidelines and document your decisions.
Building your offer (a simple playbook)
- Define your niche: (e.g., "Primary care triage workflows," "Paediatric prescribing safety," "Radiology AI evaluation").
- Assemble a one-page capability statement: List the roles you can take (e.g., CSO, evaluator, author), your areas of expertise, and your indicative day-rate.
- Join two expert networks (like GLG or Maven) for initial deal flow, then curate your own direct clients.
- Register your interest with iatroX Insights to be considered for paid SME projects aligned with your specialty.
Where to find opportunities
- Expert networks & marketplaces: GLG, Maven, Guidepoint.
- Digital-health safety: Look for firms advertising DCB0129/0160 support (like Safehand or Hardian Health) or companies in the MHRA AI Airlock / NICE EVA cohorts.
- Paid authorship: Check contributor pages for platforms like Geeky Medics or other medical media.
- Telehealth roles: National job boards like Indeed.
Red Flags to Avoid
- Low-pay annotation gigs without clear clinical impact. Be wary of platforms that treat clinical expertise as a low-wage commodity.
- Unpaid “exposure” work that requires a heavy deliverable.
- Scope creep in CSO or evaluator roles. Insist on a clear Statement of Work that defines your responsibilities, deliverables, and sign-off criteria, aligned to the DCB0129/0160 standards.
FAQs
- How common is autism among doctors?
- Community prevalence in England is ~1%; surveys of autistic doctors suggest under-diagnosis and nondisclosure make workforce figures hard to pin down.
- Is masking harmful?
- Reviews link camouflaging to anxiety, depression and burnout; safer, accommodations-first cultures reduce the need to mask.
- Can we deploy ambient voice tools now?
- Yes—only if registered and compliant with NHS guidance; all outputs need clinician verification.
- Do staff need a diagnosis for adjustments?
- No. UK guidance allows support and adjustments without a formal diagnosis.